Two centuries after its discovery, the simple yet revolutionary molecule benzene continues to shape our world. From being the gas that illuminated London in the 19th century to the cutting-edge materials it has afforded in the 21st, benzene’s journey is a compelling narrative of scientific ingenuity, industrial might, and a growing awareness of the profound responsibility that comes with chemical innovation.
In 1825, the English scientist Michael Faraday, renowned for his work in electricity and magnetism, isolated a new substance from the oily residue of the illuminating gas used to light London’s streets. When he found that it contained two parts carbon to one part hydrogen, he named it “bicarburet of hydrogen” and noted its unusual properties. (’Carburet’ denoted a compound of carbon.) It was a colourless liquid with a sweet smell. Notably, it was highly unsaturated, meaning it had a low proportion of hydrogen to carbon, which typically indicates high reactivity—yet Faraday’s new substance was surprisingly stable, resisting the addition reactions characteristic of other unsaturated hydrocarbons. It also burned with a very sooty flame, another sign of its high carbon content.
Further, when chemists soon determined its empirical formula to be C6H6, they were presented with another peculiarity. In the mid-19th century, chemists understood organic compounds as line-like chains of atoms. The concept of cyclic or ring-shaped molecules was not yet established. The empirical formula C6H6 presented a significant puzzle because a simple chain structure couldn’t account for so few hydrogen atoms relative to the number of carbons without implying a level of reactivity that benzene did not exhibit. Any proposed straight-chain structure with this formula would have numerous double and triple bonds, suggesting it should be highly reactive, but which contradicted experimental observations.
For decades, the true arrangement of these atoms remained a profound mystery, a puzzle that captivated the greatest chemical minds of the era. The breakthrough came in 1865 when the German chemist August Kekulé, famously (but also anecdotally) inspired by a daydream of a snake biting its own tail, proposed a revolutionary cyclic structure for benzene. He suggested a hexagonal ring of six carbon atoms with alternating single and double bonds. This elegant solution unlocked the door to the vast and complex world of aromatic chemistry.
The various representations of benzene.
| Photo Credit:
Vladsinger (CC BY-SA)

Rise of petrochemicals
The late 19th and early 20th centuries saw the dawn of benzene’s industrial journey. Initially extracted from coal tar, a byproduct of coke production for the steel industry, benzene found early use as a solvent and in some surprising consumer-facing applications. Due to its pleasant smell, it was even used in after-shave lotions, and for a time, it was used to decaffeinate coffee. In this era, the primary functions of aftershave were to act as an antiseptic to prevent infection from shaving cuts and to provide a pleasant fragrance. It took the mid-20th century rise of the petrochemical industry to truly unleash benzene’s potential as a fundamental building block for a vast array of materials.
Today, benzene is a crucial intermediate in the production of numerous products underpinning modern life. Petrochemicals’ primary effect on benzene was to shift its production from a limited byproduct to a commodity to be produced en masse. Benzene had until then been exclusively a byproduct of the process to use coal to make coke, which was used to manufacture steel. This meant the world’s entire supply of benzene was directly tied to the demand for steel: industrialists couldn’t just decide to make more benzene, they had to make more steel first, but that was a slow and capital-intensive process.
But with petrochemicals,the primary feedstock became crude oil and natural-gas liquids. Further, the economic boom that followed World War II, particularly the increasing use of automobiles, led to a massive expansion in refining oil to produce petrol. One process called catalytic reform could increase the octane rating of gasoline by converting linear hydrocarbons into aromatic compounds, including benzene, toluene, and xylenes (collectively known as BTX). Another process, steam cracking, heated large hydrocarbons to break them up into smaller, more useful ones, primarily ethylene and propylene (the building blocks of many plastics). A key byproduct of this process is pyrolysis gasoline, which is also rich in benzene. Benzene was thus no longer tied to steel but a co-product of the world’s largest and fastest growing industry: energy. Suddenly, its supply became abundant and scalable.
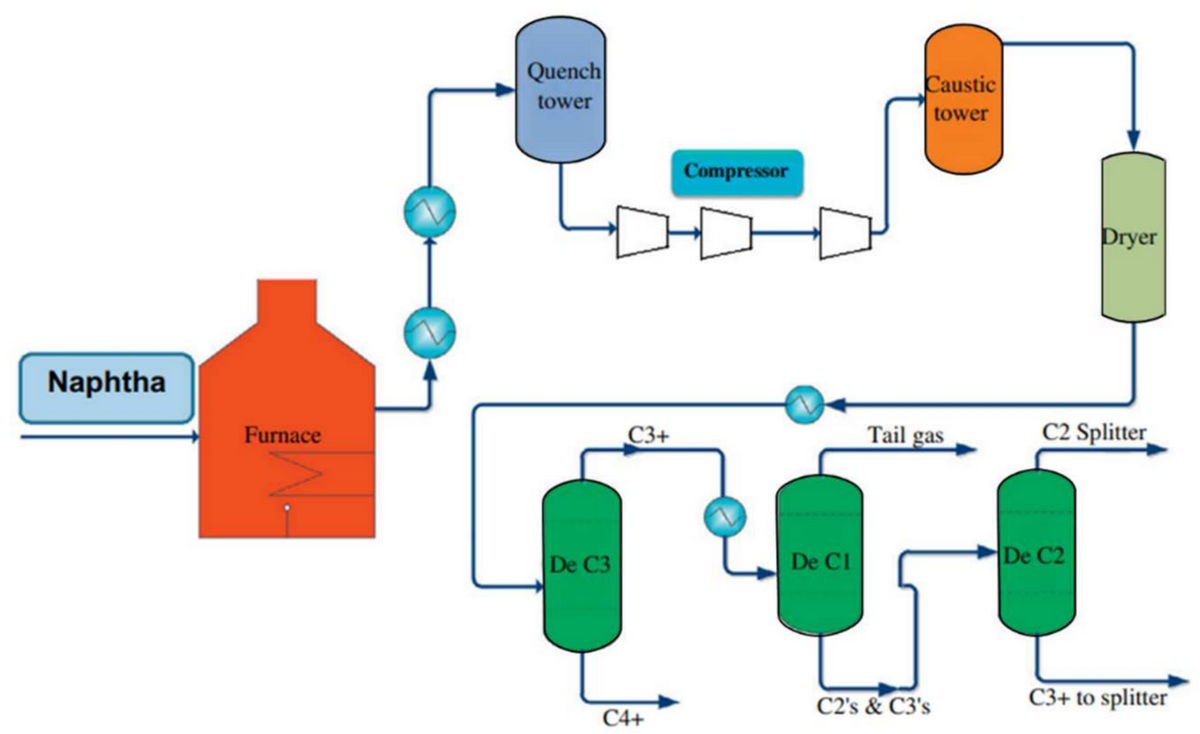
A block flow diagram of steam-cracking.
| Photo Credit:
Gholami, Z., Gholami, F., Tišler, Z., & Vakili, M. (CC BY-SA)

A steam-cracking facility in Ludwigshafen, Germany, in 2020.
| Photo Credit:
Rolf Kickuth (CC BY-SA)
Further, before the time of petrochemicals, extracting and purifying benzene from coal tar was difficult and resource-wise (relatively) inefficient. The resulting benzene also often contained impurities like thiophene, which could interfere with chemical reactions, making it unsuitable for sensitive applications like polymer production. After petrochemicals, however, benzene could access economies of scale and higher energy efficiency. Even the early petrochemical refineries were enormous and highly integrated facilities. By producing benzene on a scale hundreds of times larger than was possible with coal tar, the per-unit cost nosedived. The industry also developed highly sophisticated liquid-liquid extraction and distillation techniques to separate pure benzene from the BTX mixtures produced during reforming and cracking. As a result the industry could deliver a continuous, high-volume supply of 99%+ pure benzene.
‘Revealing prism’
This expanded availability of cheap and pure benzene created a powerful incentive for the chemical industry to find new large-scale uses for it. To them, it had gone from being a niche solvent to an abundant and affordable raw material. This directly led to the development and commercialisation of the three major chemical pathways that consume most of the world’s benzene to this day. (i) Reacting benzene with ethylene (also newly abundant from steam cracking) creates ethylbenzene, which is then converted to styrene. Styrene is the monomer for polystyrene, a versatile plastic used for everything from disposable cups and packaging foam to appliance housings. (ii) Reacting benzene with propylene (another steam-cracking product) creates cumene. This cumene process is the dominant industrial pathway to produce phenol and acetone, which are essential to make durable plastics like polycarbonates and epoxy resins. And (iii): adding hydrogen to benzene creates cyclohexane. This is the primary precursor for producing adipic acid and caprolactam, the two key ingredients for making Nylon 6 and Nylon 6,6, the fibres that revolutionised the textile industry and are also used in engineering plastics.
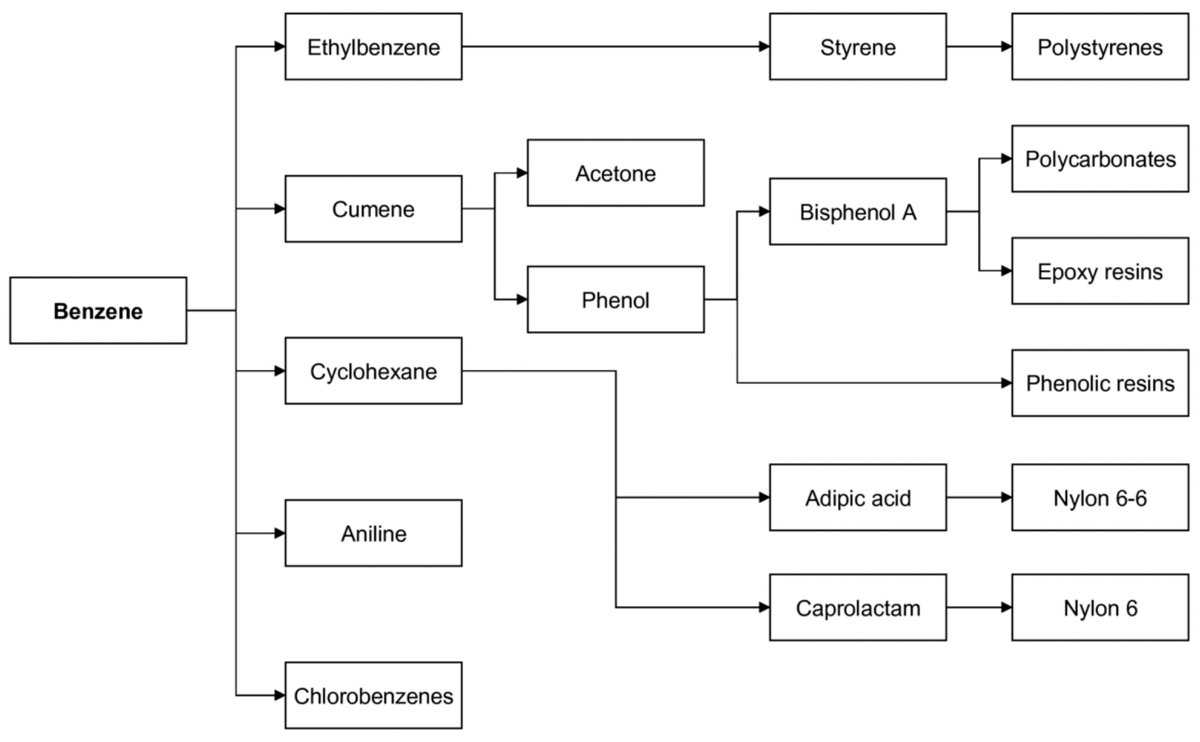
Some important commodity chemicals derived from benzene.
| Photo Credit:
Wikipedia
In essence, the petrochemical industry created a perfect, self-reinforcing ecosystem. Its own processes provided a vast supply of cheap benzene, which in turn became the essential feedstock for the very plastics and synthetic materials that drove the consumer and industrial boom of the mid-20th century. The list of benzene’s industrial offspring is extensive, including detergents, dyes, lubricants, pesticides, and pharmaceuticals. In fact, at one point, estimates suggested that two-thirds of all chemicals listed by the American Chemical Society contained at least one benzene ring.
The widespread industrial use of benzene, however, came at a significant cost. As its presence in workplaces and consumer products grew, so too did the evidence of its harmful effects on human health. The sweet-smelling liquid was, in fact, a potent toxin and a carcinogen. Early indications of benzene’s toxicity accompanied reports of aplastic anaemia and other blood disorders in workers exposed to the compound. By 1928, scientists had recognised a connection between benzene exposure and leukaemia. One 1948 study by the American Petroleum Institute concluded that the only “safe” level of benzene exposure is no exposure at all.

As historian Christopher Sellers wrote in a 2014 article: “Whereas in the 1930s the acute effects of benzene poisoning made it one of several industrial diseases recommended for workers’ compensation laws, in 1971 the growing appreciation of its chronic effects made it the first industrial chemical of the post-WWII period addressed by its own International Labour Organisation convention. Benzene’s history thus offers a revealing prism into the environmental health problems created by the global spread of modern chemical production: both the damage it inflicted on people and the checkered, gradual, and varied pathways by which the extent of this damage came to be known and regulated.”
(If you’re interested: Sellers’s article traces how scientists battled industrial denial and economic resistance and struggled for decades to prove benzene’s toxicity and carcinogenicity.)
Influence on toxicology
A gaz nozzle fit with a vapour recovery unit.
| Photo Credit:
Mroach (CC BY-SA)
Today numerous international health organisations classify benzene as a known human carcinogen: long-term exposure to it is linked to a range of life-threatening conditions, including acute myeloid leukaemia, aplastic anemia, and myelodysplastic syndrome. This growing understanding had a profound impact on both industry and research. Its use as a general purpose solvent dropped sharply in the 1980s, with safer alternatives like toluene taking its place. Regulatory bodies’ limits on benzene exposure in the workplace and on its content in consumer products like petrol also spurred innovation in closed-system manufacturing processes and the development of personal protective equipment to minimise worker exposure.
Many of the early occupational limits for benzene were often dangerously high, sometimes as much as 100 parts per million (ppm) in the mid-20th century. In 1987, the US government drastically reduced the ‘permissible exposure limit’ from 10 ppm to just 1 ppm (weighted averaged over eight hours). The US National Institute for Occupational Safety and Health recommended a limit of just 0.1 ppm, essentially treating any detectable level as hazardous. In the European Union, more recent updates (including directive no. 2022/431) have progressively lowered the binding occupational exposure limit to 0.2 ppm.
Petrol is the most significant source of benzene exposure for the general public. Historically, the benzene content in petrol could be 5% or higher by volume. In response, the US Environmental Protection Agency’s Mobile Source Air Toxics (MSAT II) rules, fully implemented by 2011, limited the annual average benzene content in gasoline at just 0.62% by volume. Similarly, the European Union Fuel Quality Directive caps benzene content in petrol to 1% by volume at best.
Before these measures, benzene vapours would often escape into the atmosphere during loading and unloading of transport vessels such as ships, railway cars, and tanker trucks. The industry responded by installing vapour recovery units, which captured these displaced vapours and either condensed them back into liquid form for reuse or safely incinerated them, preventing them from reaching workers or the environment. Traditional pumps use mechanical seals that can wear down over time, creating small fugitive emissions of benzene that are invisible but still hazardous. To meet the new standards, facilities instead adopted magnetic drive pumps, which are sealed to be airtight and have no rotating seals to leak; bellow-sealed valves later eliminated these leak points altogether. The regulations also forced refineries and chemical plants to implement rigorous leak detection and repair programmes, which often use advanced methods like infrared cameras that can ‘see’ invisible benzene plumes, allowing engineers to immediately identify and repair even the smallest leaks.
People who work with benzene need more than standardised latex or nitrile gloves: the compound can rapidly permeate these materials and be absorbed through the skin. Instead, researchers developed specialised materials for gloves and suits such as polyvinyl alcohol and fluoroelastomers like Viton, which are highly resistant to aromatic solvents. Because benzene has poor warning properties — its sweet smell is only detectable at concentrations already far above safe levels — standard dust masks or basic respirators are also insufficient. Workers in potential exposure areas must instead use full-face respirators with specific organic vapour cartridges; in higher-risk scenarios they need to don self-contained breathing apparatuses or supplied-air systems to ensure zero inhalation of the deadly vapour.

A representative image of a person wearing a full-face respirator.
| Photo Credit:
Image created with ChatGPT
In this way, the story of benzene also highlights the critical role of toxicology and occupational health research in protecting public well-being. The decades-long effort to understand and mitigate its risks led to significant advances in our ability to assess the safety of chemical compounds as well as has underscored the ethical responsibilities of the chemical industry.
Swapping atoms
As we look to the future, the narrative of benzene continues to evolve. The dual challenges of its toxicity and its reliance on fossil fuels as a primary feedstock are driving a new wave of innovation. Today, researchers are actively exploring more sustainable methods to produce benzene. One promising avenue is the development of bio-based routes, which utilise renewable feedstocks like biomass and lignin to create ‘green’ benzene (insofar as a substance like this can be ‘green’). These processes aim to reduce the carbon footprint of benzene production as well as humankind’s dependence on petroleum.
Another area of study is the chemical recycling of plastic waste to produce benzene and other valuable aromatic compounds, offering a potential solution to the growing problem of plastic pollution as well. The research and development focus is on creating safer alternatives to benzene and its derivatives, including designing novel molecules that mimic the useful properties of compounds that contain benzene while avoiding their harmful toxicological profiles. For instance, in the pharmaceutical industry, chemists are exploring the use of heterocyclic rings to replace benzene in drug candidates to improve their safety.
In benzene, the ring is formed by a closed loop of six carbon atoms. In a heterocyclic ring, one or more of those carbon atoms is swapped out for a different atom. This different atom is called the heteroatom. The most common heteroatoms in organic chemistry are nitrogen, oxygen, and sulphur. These small switches make a big difference. For example,benzene is an oil: it hates water. Many drugs need to be soluble in the bloodstream (which is mostly water) to be effective. Nitrogen and oxygen atoms can form hydrogen bonds with water, dramatically increasing a molecule’s solubility. So swapping a carbon atom for a nitrogen heteroatom can be the difference between a useless oily compound and a viable drug.
Indeed, when one of the C-H bonds in benzene is replaced with nitrogen, benzene becomes pyridine, a very common heterocycle. The nitrogen atom here has a lone pair of electrons, making it slightly negatively charged and thus basic. This allows it to act as a ‘handle’ for the molecule. A target protein or enzyme in the body can grab this ‘handle’ and form specific hydrogen bonds or ionic bonds. The heteroatom can thus allow a drug to dock into its target more precisely, like a key fitting into a lock. Further, the human body breaks down drugs primarily in the liver, using enzymes. Benzene itself is metabolised into highly reactive and toxic compounds that can damage DNA and cause cancer. By replacing the benzene ring with pyridine, chemists can often block or change these metabolic pathways. The heteroatom can make the ring more resistant to being broken down or it can guide the metabolism to produce harmless byproducts.
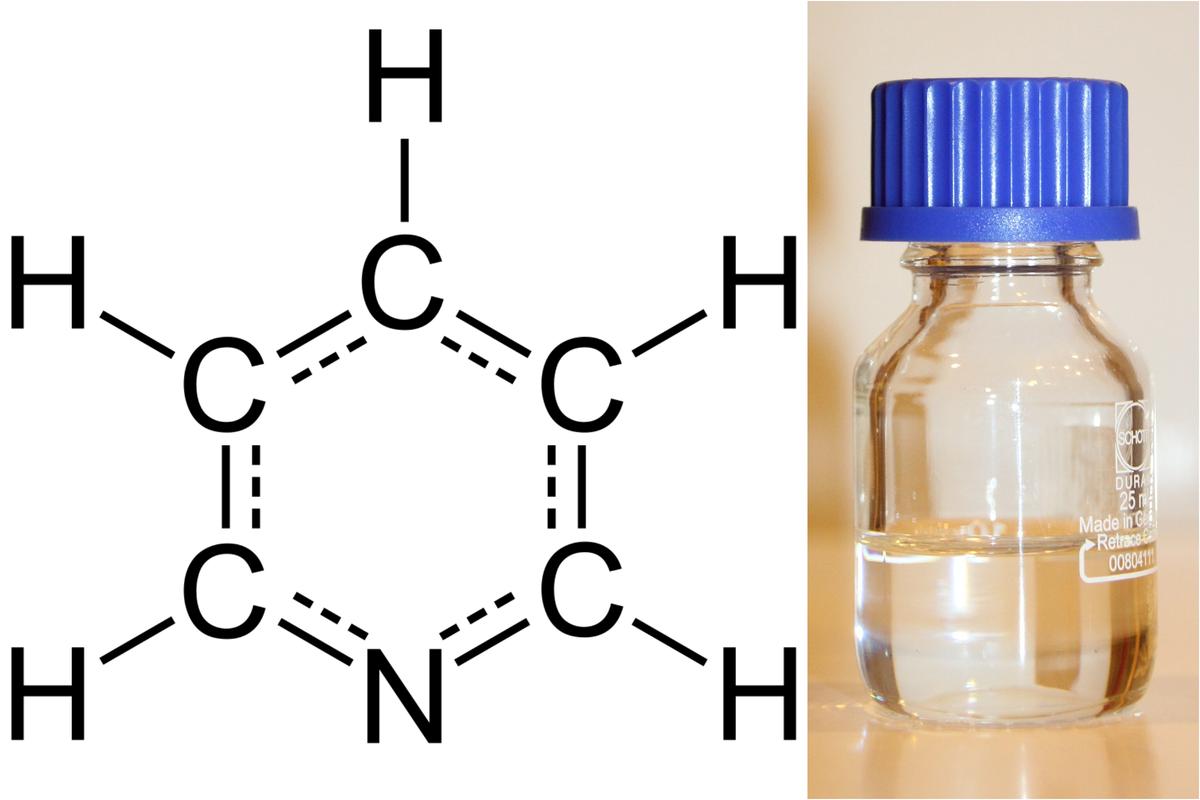
The molecular structure of pyridine (left) and a small container of the molecule as a colourless liquid.
| Photo Credit:
Jynto, LHcheM

Cautionary tale
Furthermore, benzene and its derivatives are also finding new applications in advanced materials and technologies. Chemists are investigating benzene-based polymers for use in high-performance batteries and lightweight materials that can bear high heat — all properties prized in the aerospace industry. The unique electronic properties of the benzene ring also make it a subject of interest in the development of conducting polymers and other advanced electronic materials.
Each carbon atom in the benzene ring has a spare electron residing in a p-orbital, which sticks out above and below the flat plane of the ring. Instead of forming three distinct double bonds, these six electrons merge into a single, continuous system. They are said to be delocalised, meaning they’re no longer associated with any single carbon atom but are shared equally by all six. This phenomenon creates two donut-shaped clouds of electrons, one above the ring and one below. These electrons are not fixed in place but are free to move anywhere within these donuts.
Thanks to this cloud of mobile, easily accessible electrons, a single benzene ring is an insulator but rings linked together to build larger materials can have more diverse electronic properties. For example, the primary challenge in creating a conducting polymer is to make a path for electrons to travel over long distances. Scientists achieve this by creating a ‘road’ that extends the electron highway of a single benzene ring along a long polymer chain. They start by linking benzene rings together in a chain: if they’re linked in a specific way, with alternating single and double bonds connecting the rings, the individual electron clouds of each ring can overlap and merge. This creates a continuous, delocalised electron system that runs the entire length of the polymer chain, like an electron superhighway.
In its pure state, this polymer will be a semiconductor, not a true conductor. To make it highly conductive, it has to be doped, meaning a chemical agent must be added that either removes electrons from the chain or adds extra electrons to it. Removing an electron from a location creates a positively charged hole in that spot. An electron from a neighbouring part of the chain can easily jump into this hole, leaving behind a new hole at its original location. In this way, a hole can effectively move along the chain, and this movement of positive charge is a form of electrical current. Adding an electron can likewise create a mobile negative charge that can also travel along the chain, inducing a current. This is how we have the polymer polyaniline, whose conductivity can be switched on and off by changing the acidity, making it useful for chemical sensors and anticorrosion coatings. The polymer poly(p-phenylene vinylene), or PPV, is made of benzene rings joined by short double-bonded segments. It not only conducts electricity but also emits light when a current is passed through it, making it a foundation for the development of polymer LEDs.

OLEDs can be made to be flexible, with transparent varieties finding use in foldable smartphones. Representative image.
| Photo Credit:
Ka Kit Pang

Indeed, the same principle of harnessing the mobile electrons is central to a new generation of organic electronics: devices that are flexible, lightweight, and can be manufactured cheaply. Organic light-emitting diodes (OLEDs) are the most commercially successful application. The screens on most high-end smartphones and TVs are OLEDs. In an OLED, thin films of specially designed organic molecules, many containing benzene rings, are sandwiched between electrodes. When a voltage is applied, it injects positive holes and negative electrons into the organic layer. When they meet, they release their energy as a photon of light. The specific color of the light can be tuned by changing the chemical structure of the molecule.
Organic field-effect transistors (OFETs) are the building blocks of plastic electronics, acting as the switches and amplifiers that are fundamental to computing. Pentacene, which has five benzene rings fused together in a line, is used as the semiconducting layer. While not yet as fast as silicon, OFETs can be printed onto flexible plastic substrates, opening the door for applications like smart labels, flexible displays, and wearable medical sensors. Organic photovoltaics are essentially OLEDs in reverse. Organic molecules containing benzene rings are designed to be excellent light absorbers. When sunlight strikes the material, it excites the mobile electrons, creating an electron-hole pair that can be separated and collected as an electrical current. The goal is to create cheap, flexible, and even transparent solar cells that can be integrated into windows or fabrics.
Two hundred years after its discovery, benzene remains a molecule of profound importance and complexity. Its journey from a curious byproduct of streetlighting to being a linchpin of the global economy is a testament to the power of chemical science to transform our world. Yet its tale is also cautionary—a reminder of the critical need to balance innovation with a deep and evolving understanding of the potential consequences.
